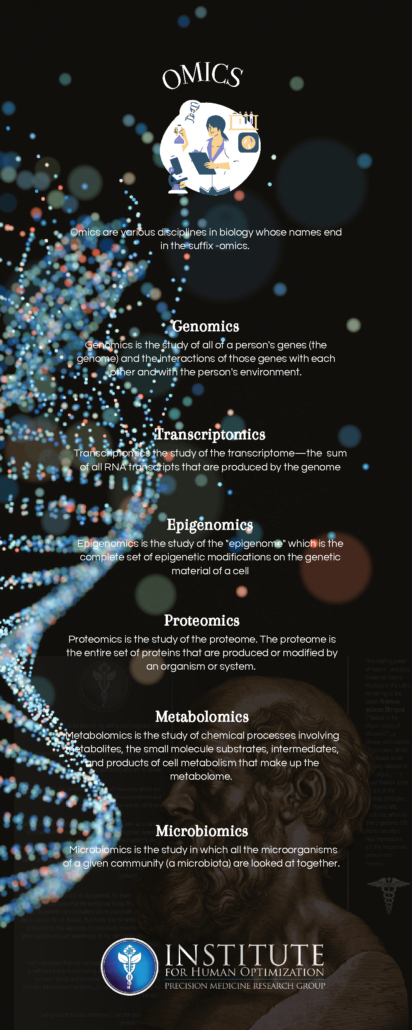
Every cell in the body is protected by a membrane. Cell membrane creates a protective barrier that shields the outside elements from the internal components of the cell, organelles. By understanding the cell membrane and what it needs, we can support our cells and cell membranes and consequently optimize our overall wellness.
The cell membrane has been historically characterized as having a fluid-mosaic model which allowed for selective permeability of various agents into the cell. Importantly, cell membrane integrity is essential to cell viability and function. Cell membranes are responsible for maintaining electrochemical gradients with a negatively charged intracellular composition and positively charged extra-cellular environment. This gives rise to what is known as the Resting Membrane Potential (RMP) and is responsible for every single physiological event that takes place within the body. Additionally, these dynamics are a key factor in the rate of aging especially within brain cells, known as neurons.
Cell membrane structure and its function.
Cells are the building blocks of life. We each have trillions of cells throughout our bodies that provide structure for the human body, take in nutrients, convert nutrients into energy, and perform specialized functions. The cell membrane, also known as the plasma membrane, is the envelope lining of the cell that shields the cell but carries important functions.
Cell membrane provides vital functions in the maintenance of cell activities including:
• They protect from toxic substance out of the cell
• Contain pathways that allow specific molecules to enter and leave the cell such as ions, nutrients, waste via transmembrane proteins.
• Separate vital metabolic processes conducted within little organs known as organelles.
• Communication
• Signal generation
Cell membranes are made up of proteins, and fats, also known as lipids. Lipids form the building blocks of cellular membranes with phospholipids being the most abundant type of lipid found in the membrane. Phospholipids are what support the cell membranes unique structure due to their hydrophobic (non-polar) tails and hydrophilic heads (polar). This means that heads of the molecules face outward and are attracted to water whereas the tails face inside away from the water allowing them to arrange themselves in a sphere form in aqueous solutions.
Cholesterol is another cell membrane component. We often only hear about how cholesterol can build up in your arteries and cause heart disease but it’s important to acknowledge its function. Biologically speaking, cholesterol is critical for cell function and plays a vital role in membrane fluidity which is the ease with which lipids move within the bilayer of the cell membrane. About 25-30% of lipid in the cell membrane is cholesterol.
Role of Phospholipids
As previously mentioned, phospholipids play a critical role in insulating cell membranes. Two of the most important outer and inner leaflet phospholipids are phosphatidylcholine (PC) and Phosphatidylserine (PS). Supplementing with phospholipids is a part of a clinical strategy known as membrane lipid replacement and is a prudent measure in maintenance of overall cellular health and aging.
Role of lipids
In addition to phospholipid compounds, there is a select class of lipids, known as the Eicosanoids, that are liberated from the membrane, metabolized into an intercellular communication and information system by their prostaglandin regulatory activity. Prostaglandins, thromboxanes, and leukotrienes mediate inflammatory signaling and coagulation pathways within the blood.
Role of Protein
Proteins are the second major component of cell membranes. Proteins mostly contribute to the function of cell membranes but also play a small role in forming the structure of the membrane.
There are 3 main types of membrane proteins
1. Integral Membrane Proteins: also known as transmembrane proteins allow molecules such as nutrients and waste to enter and leave the cell and also transmit signals between the cells internal and external environments.
2. Peripheral Membrane Proteins: form a temporary attachment with the cell membrane by allowing the proteins to attach, detach, and reattach.
3. Lipid-anchored Proteins: anchor the protein to either side of the cell membrane promoting the function of the protein to which it is anchored to.
Proteins are what help the cells interact with its environment but also help transporting substances across the cell membrane.
How to optimize cell membrane health
Now that we have a better understanding of the cell membrane structure, we can see how cell membrane integrity influences our overall health. Optimal health begins with an optimally functioning cell membrane structure. It is important to maintain a nutrient rich diet, antioxidants, and healthy fatty acids so that the cell membrane remains flexible to transport nutrients into your cells while eliminating the cells of waste. Additionally, a nutrient rich diet and healthy fatty acids is necessary for the nervous system and cardiovascular system and more.
What else can you do? Follow us along as we take a deep dive on cell membrane health during our Cell Membrane Series.
More about The Institute for Human Optimization
At the Institute for Human Optimization, we are committed to helping you create a personalized plan for living your longest, healthiest life possible. My team and I leverage the most cutting-edge advances in genetic testing, nutritional analysis, and functional medicine to get to the root biological imbalances that cause aging.
The Institute for Human Optimization was created with the intention of pursuing a highly personalized approach to longevity medicine to help enhance healthspan. Where lifespan is the actual number of years we’re alive, healthspan is how many of those years are spent in health and wellness.
We believe that a long healthspan – not just a long lifespan – is the most important thing you can cultivate. A long healthspan means you don’t miss out on life as you get older. It means remaining independent and having the vitality to travel and see the world. A long healthspan means that you can be there – in full body and mind – for the people who need you the most and that every day will feel like a gift.
We know that each person is truly unique. From DNA to iris, we all possess a blueprint that is genetically inherited and environmentally influenced. By gaining a deeper appreciation for the person on a molecular level and addressing the root causes driving disease, we can help promote optimized health through our unique scientific, N of 1, approach to individualized care.
The Institute for Human Optimization provides the most comprehensive, data-driven, personalized approach to wellness. It is:
· Predictive – We use genomics and advanced biomarker testing to risk stratification and empowerment.
· Personalized – We use data-driven health information to curate actionable change for disease mitigation and prevention.
· Preventive – We utilize highly individualized programs tailored to your unique genomic blueprint.
· Participatory – We empower engagement in personal choices, which allows for improved outcomes and enhanced results.
I am so excited about the possibility to support you on this cutting-edge journey to extend your lifespan AND your healthspan. Click here to schedule Your Longevity Equation Epigenetic Consult! Can’t wait to meet you!