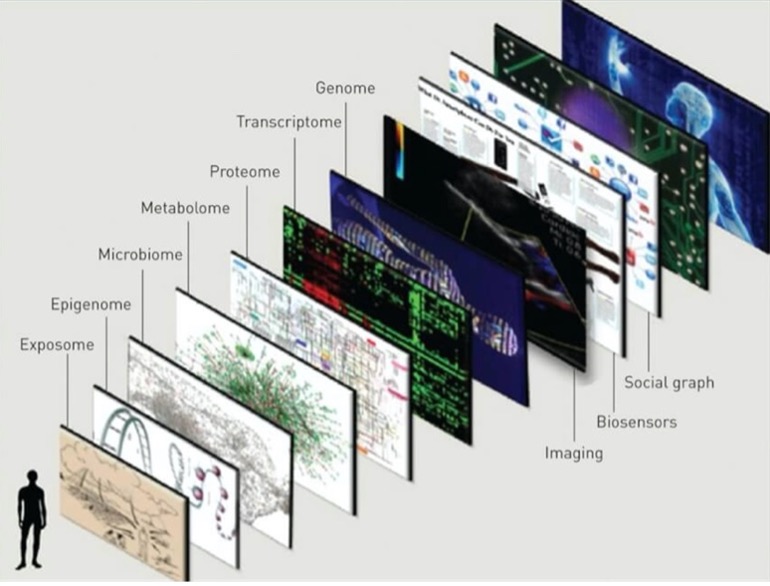
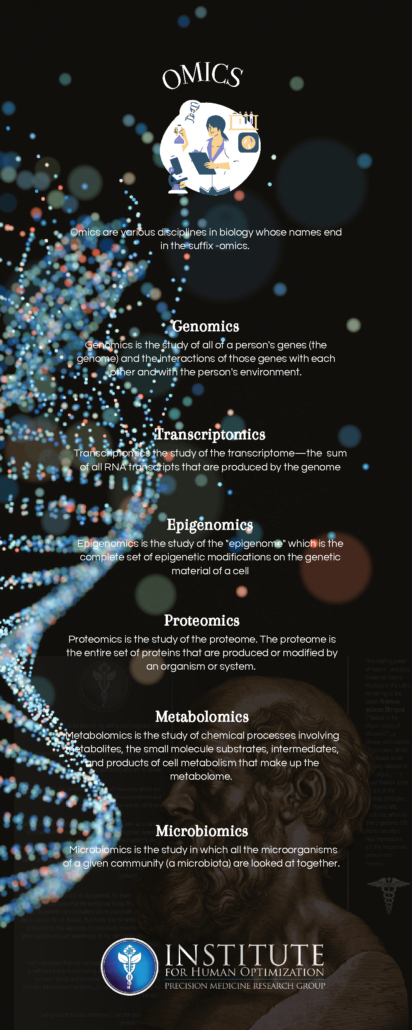
Today, we continue our series on the Omics of Medicine. If you have followed along, you will find that each Omic plays a key role in precision medicine.
This week we are taking on an important Omics system: Metabolomics. While appreciated for its role in biomarker discovery, metabolomics has emerging applications towards personalized medicine, precision nutrition research, and even agriculture.
. . .
What is metabolomics, the metabolome, and more?
Metabolomics is the large-scale study of small molecules, commonly known as metabolites, within cells, biofluids, tissues, or organisms. Metabolites are small molecules that are the end product of a metabolic process. Metabolites have several functions including but not limited to epigenetic influencing, fuel, structure, signaling, defense, and more!
Metabolites are either primary or secondary.
Primary: directly involved in normal growth, development, and reproduction of an organism. Examples include: Ethanol, Glutamic Acid, Acetic Acid, Glycerol..
Secondary: Is not involved in normal growth, development, and reproduction but serves
an ecological function and its absences is detrimental to the organism. Examples include: Peptides, Growth Hormones, Antibiotics, Alkaloids, and more.
In humans, there are thought to be approximately 3000 common metabolites although researchers suspect that there are much more. The metabolome is the complete set of metabolites within a cell. Within a cell many reactions take place such as:
-Binding
-Dissociation
-Degradation
-Modification
-Classic biochemical reaction
-Transport
These reactions are considered to be anabolic, where you are growing and/or building, or catabolic which is when you break down food and molecules for energy.
Importance of Metabolomics
The metabolome is considered the closest link to the phenotype and thus, a critical omics discipline in the pursuit of personalized medicine. How? Recall that a phenotype is an individual’s observable traits (e.g, height, eye color, blood type). These traits are mostly determined by your genotype (your set of genes) or by the environment. A phenotypic variation is due to a variation in the genotype, the environment, and how the genotype and environment interact. Omics research is currently exploring how genetic effects on phenotype are being filtered through the metabolome, making it a critical omic to possibly bridge the gap between genotype and phenotype. This will be a powerful tool in informed decision-making, developing drugs, and preventative healthcare.
Uses Today & Future Applications
Precision Nutrition: Metabolomics has emerging applications with precision nutrition which has the goal of customized nutritional recommendations. Utilizing metabolomics data, researchers can make better nutritional recommendations. For example, utilizing metabolomic data, when we look at infant formula, it has been optimally formulated to mimic the molecular composition of human milk taking into account the distribution of fatty acids.
Dietary Analysis: Researchers are using metabolites found in blood and urine to accurately measure dietary intake. This is a gateway to assess eating habits as currently the tools being used rely on our recordation and subject to human error.
Biomarker Discovery: Human metabolites being profiled has accelerated biomarker discovery.
Future of Medicine
Utilizing Omics data will provide us the lens for the molecular microscope to objectively examine individual variability. With metabolomics being used to identify metabolites that alter a phenotype, it is no surprise that many researchers consider metabolomics as the omics discipline closest to the phenotype.
The future of medicine is a precision base medicine approach that takes into account your bio-individuality, environment, lifestyle, and molecular phenotype to find the best clinical care option for you. The Institute for Human Optimization is committed to helping you create a personalized plan for living your longest, healthiest life possible. My team and I leverage the most cutting-edge advances in genetic testing, nutritional analysis, and functional medicine to get to the root biological imbalances that cause aging.
We know that each person is truly unique. From DNA to iris, we all possess a blueprint that is genetically inherited and environmentally influenced. By gaining a deeper appreciation for the person on a molecular level and addressing the root causes driving disease, we can help promote optimized health through our unique scientific, N of 1, approach to individualized care.
The Institute for Human Optimization provides the most comprehensive, data-driven, personalized approach to wellness. It is:
· Predictive – We use genomics and advanced biomarker testing to risk stratification and empowerment.
· Personalized – We use data-driven health information to curate actionable change for disease mitigation and prevention.
· Preventive – We utilize highly individualized programs tailored to your unique genomic blueprint.
· Participatory – We empower engagement in personal choices, which allows for improved outcomes and enhanced results.
I am so excited about the possibility to support you on this cutting-edge journey to extend your lifespan AND your healthspan. Click here to schedule Your Longevity Equation Epigenetic Consult! Can’t wait to meet you!